Methodology in organic synthesis
In Situ Formation of Cationic π-Allylpalladium Precatalysts in Alcoholic Solvents: Application to C–N Bond Formation
ACS Catalysis, 2022, 12, 1, 560-567
We report an efficient Buchwald–Hartwig cross-coupling reaction in alcoholic solvent, in which a low catalyst loading showed excellent performance for coupling aryl halides (I, Br, and Cl) with a broad set of amines, amides, ureas, and carbamates under mild conditions. Mechanistically speaking, in a protic and polar medium, extremely bulky biarylphosphine ligands interact with the dimeric precatalyst [Pd(π-(R)-allyl)Cl]2 to form the corresponding cationic complexes [Pd(π-(R)-allyl)(L)]Cl in situ and spontaneously. The resulting precatalyst further evolves under basic conditions into the corresponding L-Pd(0) catalyst, which is commonly employed for cross-coupling reactions. This mechanistic study highlights the prominent role of alcoholic solvents for the formation of the active catalyst.

Diastereoselective Synthesis of Nonplanar 3-Amino-1,2,4-oxadiazine Scaffold: Structure Revision of Alchornedine
Journal of Organic Chemistry, 2020, 85, 23, 15347-15359
Herein, we report the diastereoselective synthesis of a 3-amino-1,2,4-oxadiazine (AOXD) scaffold. The presence of a N–O bond in the ring prevents the planar geometry of the aromatic system and induces a strong decrease in the basicity of the guanidine moiety. While DIBAL-H appeared to be the most efficient reducing agent because it exhibited high diastereoselectivity, we observed various behaviors of the Mitsunobu reaction on the resulting β-aminoalcohol, leading to either inversion or retention of the configuration depending on the steric hindrance in the vicinity of the hydroxy group. The physicochemical properties (pKa and log D) and hepatic stability of several AOXD derivatives were experimentally determined and found that the AOXD scaffold possesses promising properties for drug development. Moreover, we synthesized alchornedine, the only natural product with the AOXD scaffold. Based on a comparison of the analytical data, we found that the reported structure of alchornedine was incorrect and hypothesized a new one.

Minimizing HCN in DIC/Oxyma-Mediated Amide Bond-Forming Reactions
Organic Process Research & Development 2020, 24, 7, 1341-1349
Aiming at advancing protocols for safer, environmentally sensible peptide synthesis we report our findings with regard to the occurrence of hydrogen cyanide (HCN, prussic acid) in amide bond-forming reactions mediated by diisopropylcarbodiimide (DIC) and ethyl (hydroxyimino)cyanoacetate (Oxyma). We determined that HCN is always formed in amide bond-forming reactions on solid support in N,N-dimethylformamide (DMF) when DIC/Oxyma is employed. In an attempt to minimize the formation of prussic acid by means of preventing the linear DIC/Oxyma adduct 2 from cyclizing to oxadiazole 3 and in turn releasing HCN, we evaluated a series of greener solvents such as N-butylpyrrolidinone (NBP), NBP/ethyl acetate (EtOAc, 1:1), methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate (PolarClean, PC), and PC/EtOAc (1:1). We found that the ratio between 2 and 3 greatly depends on the solvent used, and consequently, we further examined DMF, NBP, NBP/EtOAc (1:1), and NBP/EtOAc (1:4) as solvents for DIC/Oxyma-mediated amidations on solid support and in solution. We found that using carboxylic acid/Oxyma/DIC in a 1:1:1 ratio the rate of HCN formation decreases in the following order DMF > NBP > NBP/EtOAc (1:1) > NBP/EtOAc (1:4), while the reaction rate increases in the order of DMF ≈ NBP < NBP/EtOAc (1:1) < NBP/EtOAc (1:4). Of the solvents examined, the NBP/EtOAc (1:4) mixture gave the lowest rate of HCN formation and the highest rate of amide bond formation both in solution and on solid support. As altering the solvent for DIC/Oxyma-mediated amidations resulted in suppressing HCN rather than its full elimination we evaluated the concept of in situ scavenging of the HCN formed. We performed DIC/Oxyma-mediated amidation of Fmoc-Gly-OH + (S)-(−)-1-phenylethylamine in deuterated DMF with 0, 5, and 10 equiv of dimethyl trisulfide (DMTS) as HCN scavenger. The formation of HCN and rate of amidation was monitored by 1H NMR, revealing that DMTS scavenges HCN without inhibiting the rate of amidation. DIC/Oxyma-mediated amidations of Fmoc-Ser(t-Bu)–OH with (S)-(−)-1-phenylethylamine in DMF and NBP/EtOAc (1:4) with and without 10 equiv of DMTS were performed and found to be comparable.
POxAP Precatalysts and the Negishi Cross-Coupling Reaction
Synthesis 2020, 52, 1, 51-59
Recently developed for the Fukuyama reaction, post-oxidative addition precatalysts (POxAPs) are also very efficient in catalyzing Negishi cross-coupling reactions between organohalides and organozinc reagents. Using very low catalyst loadings, POxAPs show similar catalytic activities to those of classical precatalysts such as XPhos Pd G4 or PEPPSI-IPr, with turnover numbers of up to 93,000. POxAPs are easily prepared, are stable to air and moisture, tolerate a wide range of functional groups in the Negishi cross-coupling reaction and contribute advantageously to the arsenal of organic chemists in terms of Pd precatalysts.
From Electronic Waste to Suzuki−Miyaura Cross-Coupling Reaction in Water: Direct Valuation of Recycled Palladium in Catalysis
ChemSusChem (2020) DOI: 10.1002/cssc.202001155
From electronic waste to Pd-catalyzed reaction! The straightforward valuation of palladium recovered from electronic waste is reported here. Following a classical leaching stage, palladium is selectively extracted from a complex aqueous mixture of metallic cations into an organic phase. Afterwards, the judicious choice of a surfactant enables stabilization of palladium during back extraction cycles, and the direct preparation of an aqueous micellar solution, which can be employed in a model Suzuki−Miyaura cross-coupling reaction. Clean phase separation is observed, and distribution of all components between organic and aqueous phases is mastered. The proposed process avoids several waste generating steps dedicated to palladium isolation and ultimate purification, as well as the preparation of palladium pre-catalyst. This novel approach enables a better use of both natural resources and industrial wastes, through new cycles in circular economy.

Fukuyama Cross-Coupling Approach to Isoprekinamycin: Discovery of the Highly Active and Bench-Stable Palladium Precatalyst POxAP
Organic Letters 2019, 21, 3, 844-848
An efficient and user-friendly palladium(II) precatalyst, POxAP (post-oxidative-addition precatalyst), was identified for use in Fukuyama cross-coupling reactions. Suitable for storage under air, the POxAP precatalyst allowed reaction between thioesters and organozinc reagents with turnover numbers of ∼90000. A series of 23 ketones were obtained with yields ranging from 53 to 99%. As proof of efficacy, an alternative approach was developed for the synthesis of a key precursor of the natural product isoprekinamycin.
On water N-arylation of oxetanylamines for the preparation of N-aryl-oxetanylamines; potentially useful aryl-amide isosteres
Chem. Comm. (2019) 55, 1623-1626 (DOI : 10.1039/C8CC09110B)
A Pd cross-coupling approach for the synthesis of N-aryl-oxetanylamines has been developed. This method provides new building blocks potentially useful in medicinal chemistry as amide bioisosteres. The reactions are conducted in water employing the renewable feedstock surfactant TPGS-750-M.

Dioxygenation of styrenes with molecular oxygen in water
Tetrahedron Letters 2018, 59, 15, 1465-1468
Employing Triton X-100 as a surfactant, the tert-butyl hydroperoxide-mediated dioxygenation of styrene with molecular oxygen and N-hydroxyphthalimide was achieved in water at room temperature, providing the corresponding dioxygenated products in 9–93% yield. This facile method is eco-friendly, feasible on gram scale, and applicable to a wide range of styrene derivatives with a variety of functional groups.
Efficient and Mild Ullmann‐Type N‐Arylation of Amides, Carbamates, and Azoles in Water
Chemistry, A European Journal 2017, 23, 55, 13676-13683
A simple, sustainable, efficient, mild, and low‐cost protocol was developed for d‐glucose‐assisted Cu‐catalyzed Ullmann reactions in water for amides, carbamates, and nitrogen‐containing heterocycles. The reaction was compatible with diverse aryl/heteroaryl iodides, giving highly substituted pyridine, indole, or indazole rings. This method offers an attractive alternative to existing protocols, because the reaction proceeds in aqueous media, occurs at or near ambient temperature, and provides the N‐arylated products in good to high yields.
d‐Glucose: An Efficient Reducing Agent for a Copper(II)‐Mediated Arylation of Primary Amines in Water
ChemSusChem 2016, 9, 3244-3249
A copper‐catalyzed Ullmann‐type amination with primary amines in water with a combination of copper(II) triflate [Cu(OTf)2], dipivaloylmethane, and d‐glucose is reported. The mild conditions and the use of an inexpensive catalyst as well as a renewable feedstock (d‐glucose and the surfactant TPGS‐750‐M, which is derived from vitamin E) make this protocol a safe and convenient strategy for efficient C−N bond formation. This easy‐to‐handle procedure is extremely competitive compared to palladium‐based reactions and may be used to synthesize N‐containing molecules, such as drugs or organic light‐emitting diodes (OLEDs).

Synthesis of 3-amino-3,4-dihydro-1H-quinolin-2-ones through regioselective palladium-catalyzed intramolecular cyclization
Tetrahedron Letters, 2016, 57(14), 1547-1550
In this Letter we explored the regioselective cyclization of 2-bromophenylalanine derivatives to access enantiopure dihydroquinolinones via an intramolecular regioselective Buchwald–Hartwig cyclization using Pd2(dba)3/XPhos as a catalytic system in the presence of BTPP as organic base. This procedure represents a promising strategy for the direct synthesis of bridged Phe-Xaa dipeptides.

Rapid and scalable synthesis of innovative unnatural alpha, beta or gamma-amino acids functionalized with tertiary amines on their side-chains
Organic & Biomolecular Chemistry, 2015, 13(25), 7020-7026
We report a selective ruthenium catalyzed reduction of tertiary amides on the side chain of Fmoc-Gln-OtBu derivatives, leading to innovative unnatural alpha, beta or gamma-amino acids functionalized with tertiary amines. Rapid and scalable, this process allowed us to build a library of basic unnatural amino acids at the gram-scale and directly usable for liquid- or solid-phase peptide synthesis. The diversity of available tertiary amines allows us to modulate the physicochemical properties of the resulting amino acids, such as basicity or hydrophobicity.

Highlights :En direct des laboratoires de l'institut de Chimie
Access to 4-Alkylaminopyridazine Derivatives via Nitrogen-Assisted Regioselective Pd-Catalyzed Reactions
Journal of Organic Chemistry, 2014, 79 (21), 10311 - 10322
3-Substituted, 6-substituted, and unsymmetrical 3,6-disubstituted 4-alkylaminopyridazines were prepared from a sequence of three chemo- and regioselective reactions combining amination and palladium-catalyzed cross-coupling reactions, such as reductive dehalogenation and Suzuki–Miyaura reactions. Extension of the methodology to Sonogashira reaction yielded a novel class of 3-substituted pyrrolopyridazines.

t-BuXPhos: a highly efficient ligand for Buchwald–Hartwig coupling in water
Green Chemistry, 2014,16, 4170-4178
An efficient and versatile ‘green’ catalytic system for the Buchwald–Hartwig cross-coupling reaction in water is reported. In an aqueous micellar medium, the combination of t-BuXPhos with [(cinnamyl)PdCl]2 showed excellent performance for coupling arylbromides or chlorides with a large set of amines, amides, ureas and carbamates. The method is functional-group tolerant, proceeds smoothly (30 to 50 °C) and provides rapid access to the target compounds in good to excellent isolated yields. When applied to the synthesis of a known NaV1.8 modulator, this method led to a significant improvement of the E-factor in comparison with classical organic synthesis.
Highlights :
CNRS - Lettre-Innovation
A. Dureuil, Industrie-Pharma, 2015, 85, p58
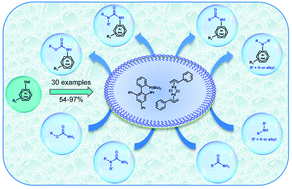
Buchwald–Hartwig reactions in water using surfactants
Tetrahedron, 2014,70, 21, 3413-3421
Examination of the scope and limitation of the Buchwald–Hartwig cross-coupling reaction in micellar medium is reported. An array of aryl or heteroaryl halides were coupled to diverse nitrogen coupling partners using a combination of [(allyl)PdCl]2 and cBRIDP to afford the corresponding products in moderate to excellent yields. 30 examples are reported, including polar solid and fairly water-soluble organic substrates/reagents.

Fully Regiocontrolled Polyarylation of Pyridine
Journal Of Organic Chemistry, 2014,79(3), 908-918
Starting from commercially available 2-chloro-3-hydroxypyridine, a new route leading to the first protypical pentaarylpyridine bearing five different substituents is reported. This strategy involves a set of five sequential but fully regiocontrolled Suzuki–Miyaura reactions and highlights the 2-OBn pyridine protecting group as a key intermediate. The 2-OBn group played a double role: (i) it allowed additional bromination at position 5 and (ii) it could afford the reactive OTf species for the last C-arylation step at the less hindered 2 position of the tetraarylpyridine. The photophysical properties of the novel compounds are also described. The synthesized pentaarylpyridine derivative exhibit a large Stokes shift, strong solvatochromism, and quantum yield values up to 0.47; thus, they constitute promising building blocks for the design of environment-sensitive probes.

Nucleophilic Substitution of Azide Acting as a Pseudo Leaving Group: One-Step Synthesis of Various Aza Heterocycles
Journal Of Organic Chemistry, 2013,78(22), 11335-11341
The reaction of 3-azidopropanoic acid with the carbodiimide-based coupling reagent DIC leads to a six-membered-ring intermediate acting as a versatile precursor to a diverse set of aza heterocycles, including mono-, bi-, and tricyclic compounds.





